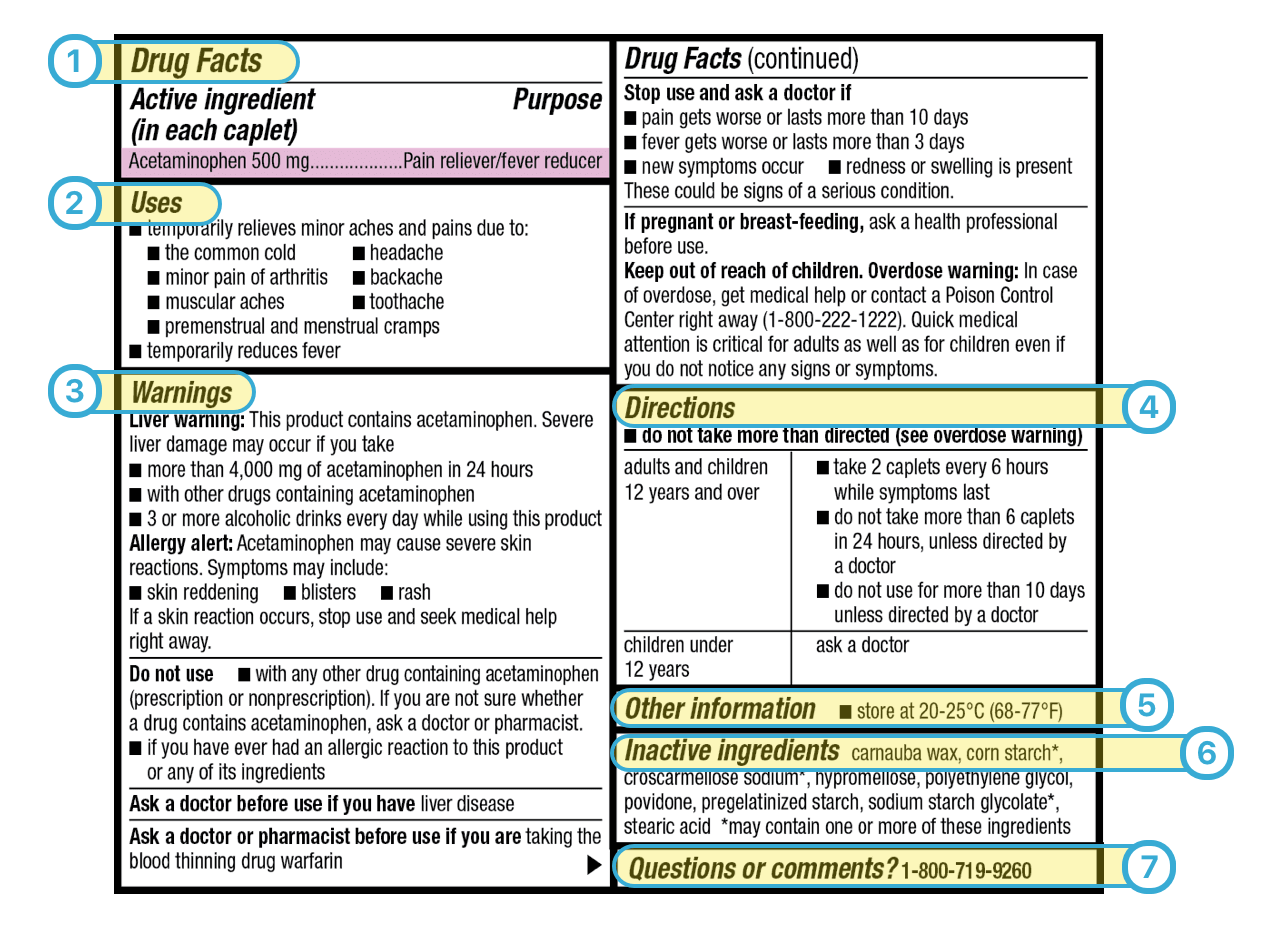
Fda Drug Facts Label Guidelines . the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. Fda required changes to the. in 2016, the u.s. This paragraph applies only to prescription. Section 201.66 standard labeling format a. (d) labeling requirements for new and more recently approved prescription drug products. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. The drug facts labeling is.
from www.drugwatch.com
Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. (d) labeling requirements for new and more recently approved prescription drug products. The drug facts labeling is. Section 201.66 standard labeling format a. This paragraph applies only to prescription. in 2016, the u.s. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. Fda required changes to the.
How to Read OvertheCounter and Prescription Drug Labels
Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. in 2016, the u.s. (d) labeling requirements for new and more recently approved prescription drug products. Section 201.66 standard labeling format a. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. Fda required changes to the. This paragraph applies only to prescription. The drug facts labeling is.
From ar.inspiredpencil.com
Fda Labeling Regulations Fda Drug Facts Label Guidelines the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. in 2016, the u.s. The drug facts labeling is. the nonprescription drug facts. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Fda required changes to the. The drug facts labeling is. in 2016, the u.s. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic.. Fda Drug Facts Label Guidelines.
From www.slideserve.com
PPT FDA LABELING PowerPoint Presentation, free download ID3633953 Fda Drug Facts Label Guidelines in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). the drug. Fda Drug Facts Label Guidelines.
From www.fda.gov
OTC Drug Facts Label FDA Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). The drug facts labeling is. (d) labeling requirements for new and more recently approved prescription drug products. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. in 2016, the u.s. . Fda Drug Facts Label Guidelines.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements Fda Drug Facts Label Guidelines Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). Fda required changes to the. This paragraph applies only to prescription. The drug facts labeling is. in the interest of uniformity of presentation,. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Fda required changes to the. Section 201.66 standard labeling format a. (d) labeling requirements for new and more recently approved prescription drug products. This paragraph applies only to prescription. in 2016, the u.s. The drug facts labeling is. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the. Fda Drug Facts Label Guidelines.
From exocyqbgg.blob.core.windows.net
Drug Facts Labels Of Us Fda Pdf at Diana Swank blog Fda Drug Facts Label Guidelines Fda required changes to the. This paragraph applies only to prescription. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. The drug facts labeling is. Section 201.66 standard labeling format a. in 2016, the u.s. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines This paragraph applies only to prescription. in 2016, the u.s. The drug facts labeling is. Section 201.66 standard labeling format a. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks.. Fda Drug Facts Label Guidelines.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). Section 201.66 standard labeling format a. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Fda required changes to the. (d) labeling requirements for new and more recently. Fda Drug Facts Label Guidelines.
From exocyqbgg.blob.core.windows.net
Drug Facts Labels Of Us Fda Pdf at Diana Swank blog Fda Drug Facts Label Guidelines in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. Section 201.66 standard labeling format a. the drug facts labeling for otc drug products is intended to make it easier for. Fda Drug Facts Label Guidelines.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Fda Drug Facts Label Guidelines The drug facts labeling is. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. This paragraph applies only to prescription. (d) labeling requirements for new and more recently. Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines The drug facts labeling is. in 2016, the u.s. This paragraph applies only to prescription. (d) labeling requirements for new and more recently approved prescription drug products. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. Fda required changes to the. Section 201.66. Fda Drug Facts Label Guidelines.
From foodindustryexecutive.com
FDA Final Guidance Clarifies New Nutrition Label Requirements Food Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). in 2016, the u.s. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. The drug facts labeling is. Food and drug administration (fda) updated requirements. Fda Drug Facts Label Guidelines.
From www.lifealert.org
OvertheCounter Medicine Label Fda Drug Facts Label Guidelines the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the. Fda Drug Facts Label Guidelines.
From exocyqbgg.blob.core.windows.net
Drug Facts Labels Of Us Fda Pdf at Diana Swank blog Fda Drug Facts Label Guidelines Fda required changes to the. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. in 2016, the u.s. Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. The drug facts labeling is. This paragraph applies only to prescription. . Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Section 201.66 standard labeling format a. Fda required changes to the. (d) labeling requirements for new and more recently approved prescription drug products. The drug facts labeling is. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. This paragraph applies only to prescription. . Fda Drug Facts Label Guidelines.
From animalia-life.club
Fda Drug Labeling Requirements Fda Drug Facts Label Guidelines Food and drug administration (fda) updated requirements for the nutrition facts label on packaged foods and drinks. (d) labeling requirements for new and more recently approved prescription drug products. in 2016, the u.s. in the interest of uniformity of presentation, fda strongly reccommends that the drug facts labeling be presented using the graphic. Fda required changes to. Fda Drug Facts Label Guidelines.
From myoldmeds.com
Read the Label Understanding the Drug Facts Label on Over The Counter Fda Drug Facts Label Guidelines the nonprescription drug facts label in a changing consumer marketplace 2021 overview of the drug facts labeling (dfl). Fda required changes to the. the drug facts labeling for otc drug products is intended to make it easier for consumers to read and understand otc drug product. The drug facts labeling is. This paragraph applies only to prescription. . Fda Drug Facts Label Guidelines.